Cleara Biotech B.V. was founded in order to translate academic findings to benefit patients. Some historical highlights that provided rationale for translation through Cleara include:
2008
FOXO4 and p53 can physically interact in cells. Both proteins are involved in how cells response to damage and stress, but were so far mostly considered to have separate biological roles. The FOXO4/p53 interaction is especially elevated under conditions of stress. Subsequently, molecular feedback loops are activated to counter the negative consequences. Phosphorylation of p53 on a particular residue can occur under such conditions – something which has become important to Cleara’s development later on.
2009
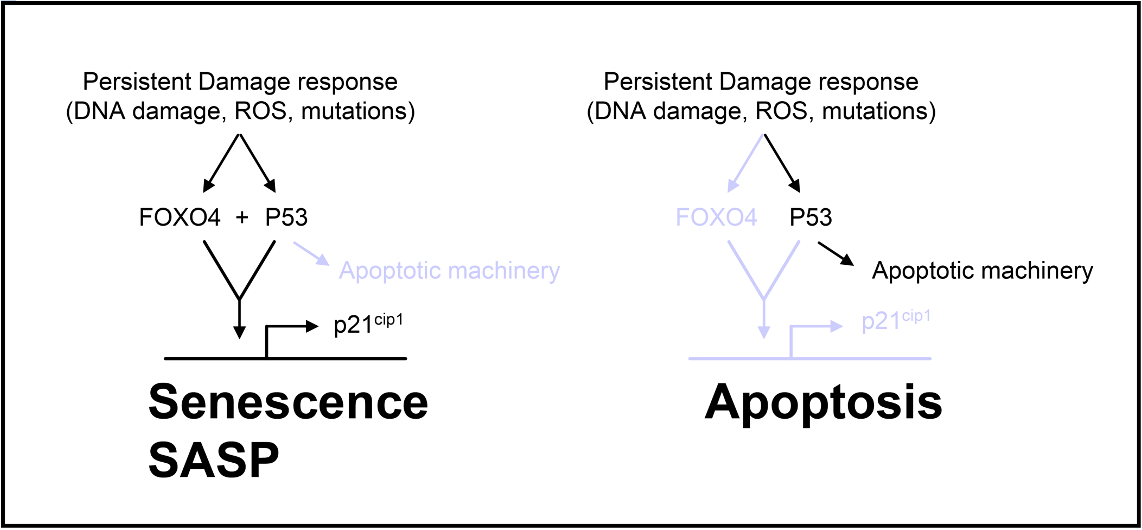
Hyperactivating mutations in proteins that promote cellular growth and/or proliferation (oncogenes) can cause a persistent form of cellular stress. Subsequently, such chronic stress signaling can cause cells to become senescent (or senescence-like, in case of cancer cells). FOXO proteins are downstream targets of at least some of these senescence responses. Under these conditions, FOXO4 phosphorylation and binding to p53 occurs. Importantly, inhibition of FOXOs in such cells tipped the balance from senescence towards apoptosis. This lead to the following model (e.g. P.L.J. de Keizer, Ph.D. dissertation and “de Keizer et al., 2010, Cancer Research”):
2010
Identification by our team that FOXO4 is a pivot in maintaining the viability of senescent cells. Using fibroblasts induced to senesce by ionizing radiation, we further observed that p53 is phosphorylated on Serine 46 and that removing FOXO4 could trigger a “clean” cell death response, known as apoptosis (de Keizer&Campisi, Cancer Res (2011) 71 (18_Supplement): C35.). This created a new window of opportunity to eliminate these deleterious cells. As we found FOXO4 to also exert a protective response to chemotherapy, it additionally opened the door towards development of methods to overcome therapy resistance of cancer cells.
2012
Generation of the first and second generation of compounds to eliminate senescent and therapy-resistant cancer cells (e.g. patent US20130288981 A1; 2012). Here, we showed for the first time that not only inhibition of FOXO4 itself, but more specifically, blocking the FOXO4-p53 binding by cell-penetrating peptides could effectively eliminate senescent cells and therapy-resistant cancer cells. A downside was that these peptides were based on regular “L” amino acids, something which we addressed next.
2015
Development of the third generation of FOXO4-based anti-senescence drugs: the FOXO4-DRI peptide. In contract to the earlier versions, this peptide fully contained “D”-amino acids. Moreover, the sequence was inverted. This 3rd generation FOXO4-DRI proved to be effective in counteracting signs of chemotoxicity and, excitingly, was able to restore healthspan in models for fast and natural aging, e.g. fur density, behavior and renal function. (Baar et al., Cell, 2017). This research received worldwide media attention, including coverage in numerous TV and radio shows, newspapers and blogs. Multiple other groups have shown benefits of FOXO4-DRI against (age-related) diseases in mouse models.
2018
Incorporation of Cleara to continue our evolution of FOXO4-based anti-senescence drugs and generate the fourth generation capable of human translation. A problem we encountered was that that “aging” is not an easy target for clinical trials. Moreover, the selectivity of the 3rd generation peptide was such that we considered improvement to be necessary.
2018-2022: Research and optimization phase
Back in 2018, Cleara realized that not all senescence is equal and some subtypes generally tend to be more problematic than other. One such subtype, we identified to be positive for cell scarring. This subtype is characterized by PML/FOXO4 nuclear foci that are adjacent to a specific form of p53 that is phosphorylated on S46, T55 and S392. Importantly, scarred cells are generally highly secretory and found in numerous diseases that Cleara is pursuing.
To optimize its 4th generation FOXO4-based peptides, Cleara engaged in multiple cycles of structural, molecular and cell biology.
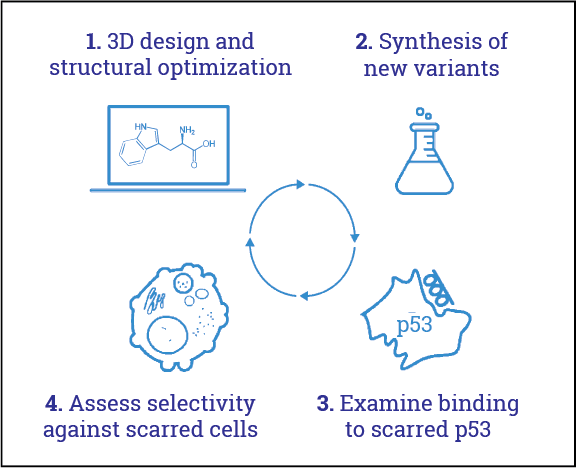
This ultimately led to two lead compounds, CL04177 and CL04183. Excitingly, and in contrast to the 3rd generation FOXO4-DRI (Cell, 2017), Cleara’s CL04177 and CL04183 show a strongly enhanced binding to p53 when it is scarred.
2022-2024: Proof of concept phase
Following in vitro development of these lead candidates, Cleara pursued several directions to assess their potency in proof-of-concept studies in vivo. Ultimately, CL04183 was nominated as “Development Candidate”. As shown in our Pipeline, CL04183 is efficacious in numerous animal models for diseases as:
– Oncology (>25% mCRC, TNBC and HGSOC qualify)
– Neurodegeneration (maintenance of cognitive + physical function until end of life)
– Liver fibrosis (maintenance of liver function in CCl4-mouse model)
– Cardiovascular health (>9 markers for heart function)
Importantly, third party experiments showed CL04183 to induce an impressive 29% overall survival increase in geriatric mice (825 days at start of treatment). Thus, CL04183 has broad potency against diseases where scarred cells are a culprit.

2024 – 2025: Preclinical development (IMPD/IND readiness)
In 2024, Cleara received a new capital injection which allowed it to pursue Pre-IND/IMPD studies for clinical translation. GLP-Tox studies we conducted with senior 3rd party CROs, which led to the conclusion that CL04183 was well tolerated in rats and non-human primates.
On the CMC side, Drug Substance work was completed and Drug Product work to a phase prior Clinicial Trial Application.
2025 onwards: Clinical trial design, application and execution
Together with our 3rd party CROs, Cleara’s senior medical and clinical team designed Phase 1a and 1b clinical trials to examine the efficacy of CL04183 in patients. While the primary readouts are safety, CL04183’s efficacy will be measured in plasma as well. This will provide a meaningful measure of efficacy in an early phase and is designed to strongly derisk the project.
For this phase, Cleara is looking for Series-A financing or partnering with pharmaceutical companies.

